60 million children in the U.S. have been diagnosed with ADHD, accounting to almost 10% of all children (1). Many groups have their theories why ADHD is on an increase in all generations, younger ones included, but an undisputed fact is that attention-deficit disorders are on the rise. The canonical treatment for the disorders are stimulants that are prescribed by the millions every year, but the majority of these molecules are Schedule II with a potential for abuse, and have the potential for misuse.
Supernus Pharmaceuticals developed viloxazine, brand name Qelbree, and have gotten approval by the FDA on April 2, 2021 (2). Viloxazine is a selective norepinephrine reuptake inhibitor that has been approved for ADHD treatment for children aged 6 to 17. Its appeal as a non-stimulant treatment for the common disorder, along with its low risk of side effects provides a compelling case over more traditional treatments.
A Phase III trial concluded that, after 8 weeks of use in children ages 6 to 11, there was a definite improvement in the ADHD Rating Scale for symptoms versus a placebo, and the rate of patients that dropped out on account of adverse effects from the treatment was less than 5% (3). A metastudy of many of the trials conducted by the company gave average improvement numbers upwards of 50% for multiple tests designed to evaluate ADHD symptoms (4).
Viloxazine was developed in the 1970’s and used in Europe as an antidepressant after being brought to the market in 1976 (5). However, it was withdrawn decades later as the SSRI’s that are now staples of the antidepressant market took over.
https://doi.org/10.2147/JEP.S256586
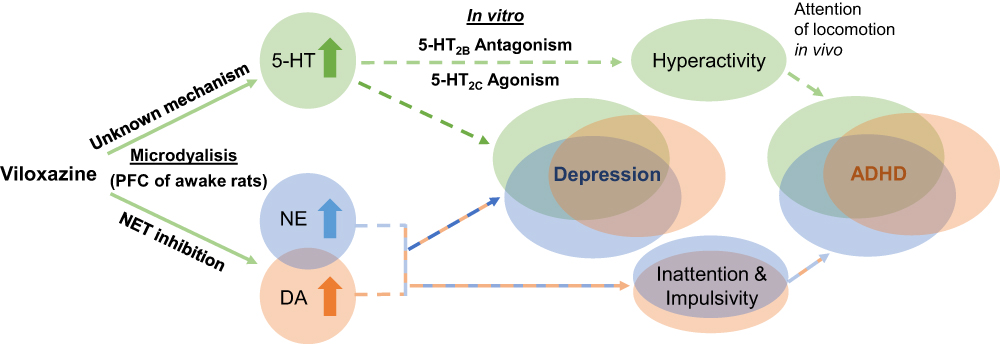
The small molecule differs from other SNRIs by possibly also up-regulating GABA-B receptors, which could lead to possibly different pharmacodynamics than other drugs in the same class, but there is much room for further research here (6).
By: Pavle Mihajlovic